Pipeline
Topical Roflumilast Cream
(ARQ-151)
How it Works
Topical roflumilast cream (ARQ-151) is a topical formulation of roflumilast, an advanced targeted topical phosphodiesterase type 4 (PDE4) inhibitor. Inhibiting PDE4, an intracellular enzyme that is an established target in dermatology, decreases the production of pro-inflammatory mediators. This decreases inflammation in the skin and balances the skin’s immune system.
Topical roflumilast cream is a highly potent, selective PDE4 inhibitor in a convenient, once-daily formulation that Arcutis is studying in a range of inflammatory skin diseases, including atopic dermatitis and plaque psoriasis.
Based on in vitro data only. The specific mechanism by which exerts its therapeutic action is not well defined.

Explore a recent FDA approval.

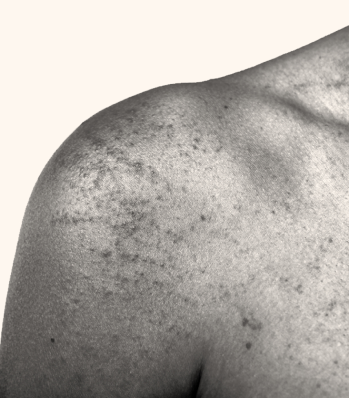
Atopic Dermatitis
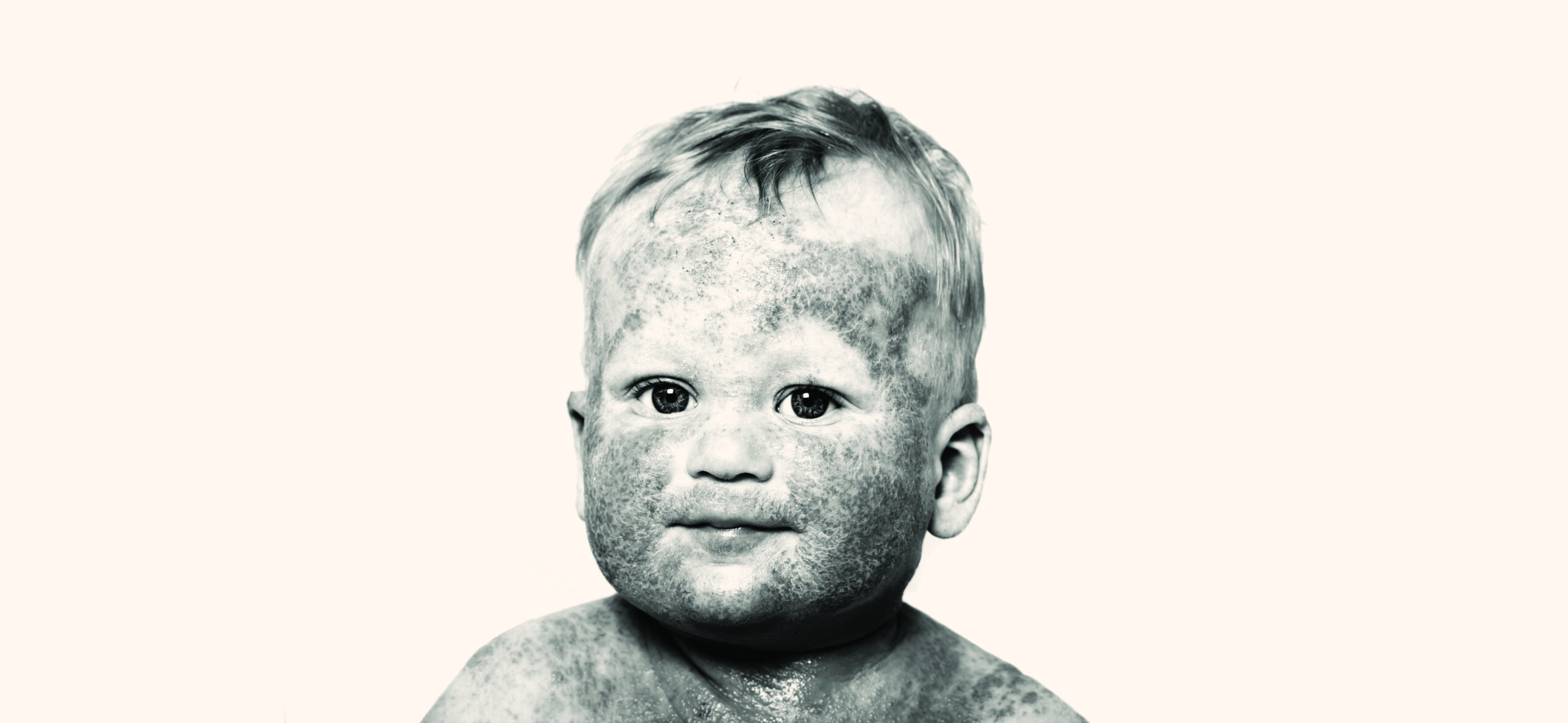
Arcutis is investigating roflumilast cream 0.05% for the treatment of atopic dermatitis in children 3 to 24 months old.
We have begun enrollment of the INTEGUMENT-INFANT Phase 2 open-label clinical study, which is evaluating the safety and tolerability of investigational ZORYVE (roflumilast) cream 0.05% in infants aged 3 months to less than 24 months with mild to moderate atopic dermatitis.
Plaque Psoriasis
Arcutis is investigating roflumilast cream 0.3% for the treatment of plaque psoriasis in children ages 2 to 5
We have submitted a supplemental New Drug Application (sNDA) for investigational ZORYVE (roflumilast) cream 0.3% to expand its indication to the treatment of plaque psoriasis in children ages 2 to 5. If approved, roflumilast cream would be the first and only topical PDE4 inhibitor indicated for plaque psoriasis in children as young as 2 years old, offering patients and caregivers an important alternative to topical steroids and vitamin-D analogs.
Arcutis’ pipeline will change as molecules move through the drug development process. The safety and efficacy of the investigational agents or investigational uses of marketed products have not been established. These uses have not been approved by the US Food and Drug Administration or other regulatory authorities.
There is no guarantee that these therapies or uses will be commercialized. Some of the content on this page is not intended for users outside the US.
Meaningful innovation
at your fingertips.
Read scientific publications.
View