Pipeline
Topical Roflumilast Foam
(ARQ-154)
Clinical development
Topical roflumilast foam (ARQ-154) is a foam formulation of a topical formulation of roflumilast, an advanced targeted topical phosphodiesterase type 4 (PDE4) inhibitor. Inhibiting PDE4, an intracellular enzyme that is an established target in dermatology, decreases the production of pro-inflammatory mediators. This decreases inflammation in the skin and balances the skin’s immune system.
Topical roflumilast foam is a highly potent, selective PDE4 inhibitor in a convenient, once-daily formulation that Arcutis is studying in several inflammatory skin diseases, including hidradenitis suppurativa (HS) and vitiligo.
Based on in vitro data only. The specific mechanism by which exerts its therapeutic action is not well defined.

Read about one of our FDA approvals.
Investigational Research
We are advancing proof-of-concept Phase 2 studies to explore the potential of ZORYVE (roflumilast) foam 0.3% for the treatment of hidradenitis suppurativa (HS) and vitiligo.

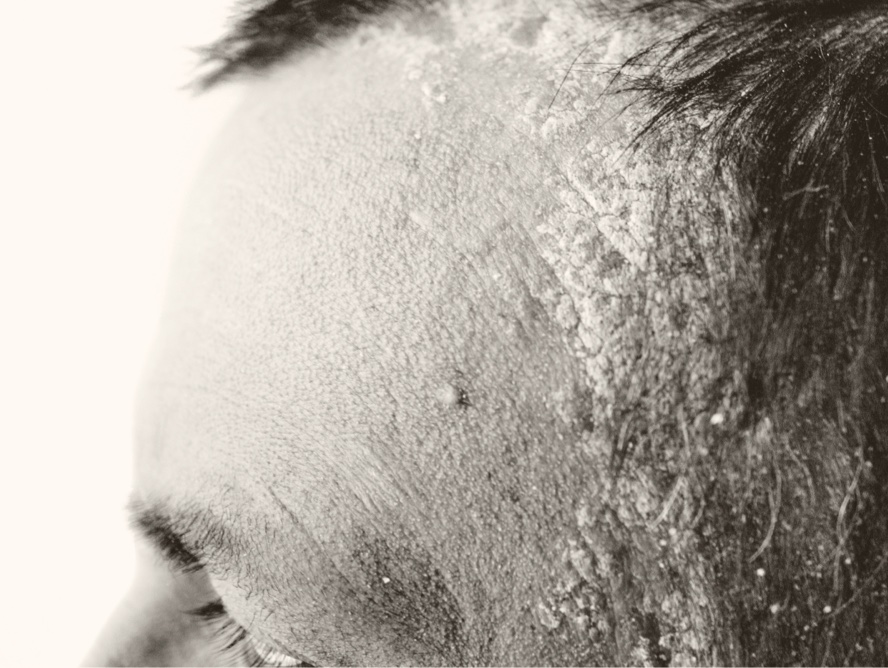
Scalp Psoriasis
Additionally, 46.5% of individuals treated with roflumilast foam achieved Body-Investigator Global Assessment (B-IGA) success at week 8 compared to 20.8% of individuals treated with vehicle. Once-daily roflumilast foam also demonstrated a favorable safety and tolerability profile. Based on this positive data, Arcutis intends to submit a supplemental NDA to the FDA.
Clinical Trials
Completed
Meaningful innovation
at your fingertips.
Read scientific publications.
View